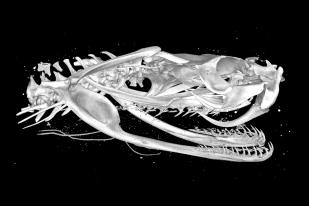
Laboratories
The laboratories of the Museum für Naturkunde Berlin provide space for integrated research. Their links to the museum’s collection, the analytical equipment and the interdisciplinary research approaches make the museum a unique research institution.
The laboratories are open to museum staff, students, doctoral students, postdocs and visiting scientists for their research projects. Contact details are given in the descriptions of the individual laboratory units:
- 3D Laboratory
- Bioacoustics Laboratory
- Collection Care Laboratories
- Geochemical and Microanalytical Laboratories
- High-Performance Computing Cluster
- Integrated Zoological Research Laboratory
- Isotope laboratory
- Mediasphere For Nature
- Molecular Genetics Facilities
- Paleontological preparation laboratories